Can a metal prized for corrosion resistance actually fail when you need it most? I ask this because many people assume that once a surface is labeled resistant, it never needs care.
I answer that stainless steel resists rust thanks to a thin, self-healing chromium oxide film. That passive layer forms when chromium (about 10.5–11% or more) bonds with oxygen on the surface. However, under certain conditions, stainless steel can still become susceptible to rusting. Factors such as exposure to chlorides, high humidity, or physical damage can compromise the passive layer, leading to what are referred to as ‘stainless steel rusting causes.’ Regular maintenance and careful selection of stainless steel grades can help mitigate these risks and preserve its integrity. Additionally, environments that contain chemicals or harsher elements can accelerate the deterioration of the chromium oxide layer, which in turn exposes the underlying metal to corrosion. Understanding what leads to stainless steel rust is essential for maintaining the longevity and aesthetic appeal of stainless steel products. By implementing protective measures and conducting routine inspections, one can effectively prevent rust formation and ensure the durability of stainless steel components.
But resistance is not immunity. In chloride-rich, acidic, or oxygen-starved crevices the film can fail and corrosion can start. I will show practical steps for selection, maintenance, and repair.
In this guide I preview the main families and where I use each in kitchens, structures, and harsh services. I also explain how simple tests for magnetism hint at alloy type and help you match grade to applications.
Read on for a clear, practical framework to prevent rust, extend service life, and choose the right materials for your needs.
My short answer and why it matters right now
My short verdict: this common alloy resists major corrosion but can still show rust when conditions turn aggressive.
Chromium in the mix builds a thin oxide layer that limits oxidation to the outer atomic layers and can self-repair when oxygen is present.
That passive film is the core of corrosion resistance, but salt spray, acidic cleaners, and high cooking heat can defeat it. Choosing the right grade matters: a 304 sink near the coast may tea-stain, while 316 or duplex performs much better in chloride-rich air and splash zones.
Routine, gentle cleaning helps the surface repassivate daily. Alloying with nickel, molybdenum, and nitrogen also reduces pitting risk in tough services.
- Right grade + simple care cuts replacement and downtime.
- Neglect lets small rust blooms signal deeper pitting or crevice attack.
- I’ll show exact steps next: cleaner choice, rinse practice, and avoiding embedded iron.
Understanding stainless steel vs. rust: how I define the problem
Let’s separate surface discoloration from active metal attack to spot real risk quickly.
I define rust here as visible iron oxide that forms where the passive chromium oxide film has failed or where free iron was introduced into the surface. That distinction matters because treatment differs for cosmetic stains and for true material loss.
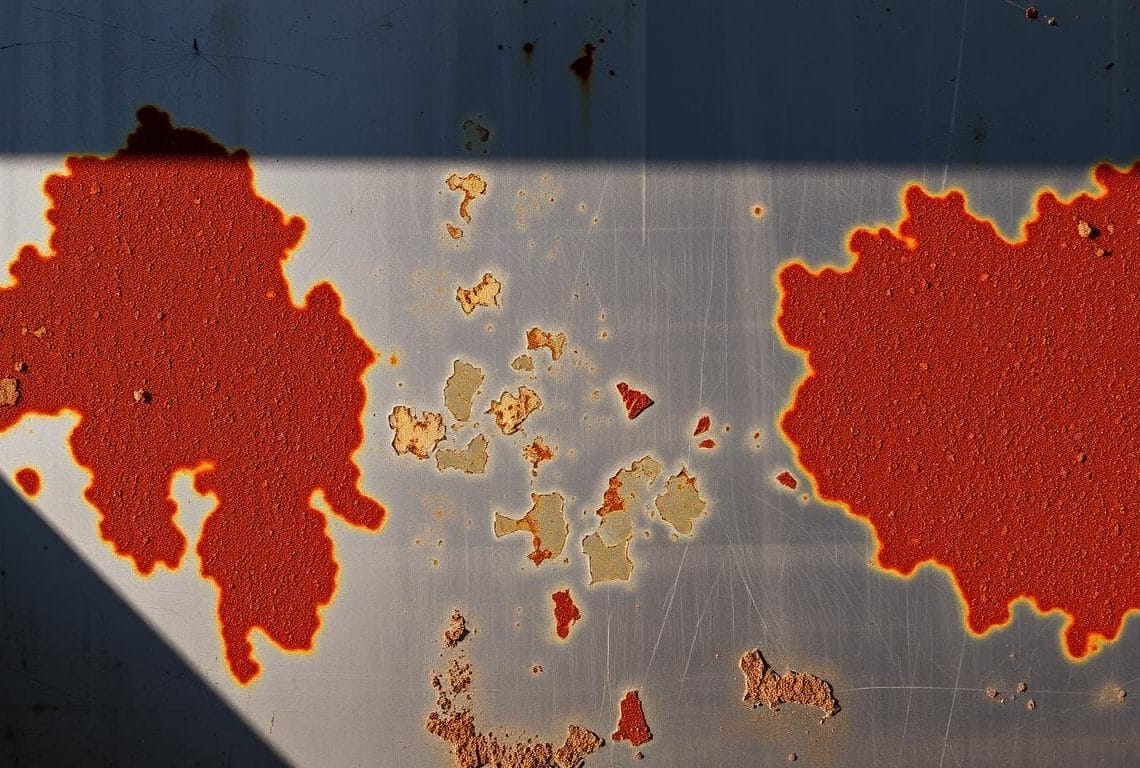
Rust and iron oxide: what you actually see on the surface
The protective oxide layer is only nanometers thick, yet it blocks oxygen transfer and protects the substrate. When that layer breaks, you may see brownish iron oxide or localized pits.
Corrosion vs. staining: knowing the difference guides the fix
Staining such as tea staining is often cosmetic and usually wiped away or polished. Corrosion like pitting or crevice attack signals metal loss and needs targeted treatment.
- I check with magnification and a wipe test to tell contamination from active attack.
- Embedded iron from fabrication or abrasive wool can smear particles and trigger localized rust.
- Oxygen-starved crevices fail to repassivate and behave differently than open surfaces.
Match symptoms to causes—chlorides, acids, heat tint, or embedded particles—to pick the right fix. For more on whether a surface will corrode and practical remedies, see my detailed guide on can stainless steel rust.
How the passivation layer protects stainless steel
A tiny, self-repairing film at the metal-air boundary controls whether an item will stain or suffer true corrosion.
Chromium and oxygen: the self-healing oxide mechanism
I rely on chromium to form a dense chromium oxide that bonds to the metal and blocks further attack. This thin layer mends itself when oxygen is available, which is why exposure to air helps maintain corrosion resistance.
When the passive film breaks down in real-world use
Chlorides, acids, tight crevices with low oxygen, high temperature scaling, and mechanical scratches all defeat the oxide barrier. Embedded carbon metal particles or contamination start localized rust sites quickly.
Passivation in practice: restoring the protective layer
I use chemical passivation to remove contaminants and enrich chromium at the surface. Electropolishing is my choice when an extra-smooth finish and higher corrosion resistance are required.
- Prep first: clean oils and free iron so the process is uniform.
- Verify: water-break tests and corrosion coupons confirm a good finish.
- Remember: passivation is a process, not a coating — exposure to air lets the film self-heal.
Families of stainless steels and where I use each
I group common alloys into five families so you can match properties to real-world tasks.
Austenitic examples and everyday use
Austenitic stainless steels (200/300 series) have an FCC structure and are usually non‑magnetic. I pick 304 for general use and 316 when chloride exposure is expected because 316 adds molybdenum for better pitting resistance.

Ferritic: cost‑aware, magnetic options
Ferritic grades are BCC and magnetic. I use them where low nickel content matters and corrosion risk is moderate, for trim and simple panels.
Martensitic: hard, wear‑resistant parts
Martensitic grades take heat treatment to harden. I specify these for blades, shafts, and wear parts when edge retention outranks maximum corrosion resistance.
Duplex: strength plus chloride resistance
Duplex mixes austenite and ferrite to boost yield strength and resistance to chloride stress cracking. I choose duplex for brackish water and splash‑zone components.
Precipitation hardening for high strength
Precipitation hardening alloys like 17‑4 PH reach high strength with heat treatment while keeping good corrosion performance. I use them in compact, load‑bearing parts.
- I weigh crystal structure, magnetism, corrosion profile, and fabrication limits.
- Match grade to environment, strength needs, and budget for reliable results.
does stainless steel rust in the real world?
Field experience shows that corrosion appears when chemistry, design, or contamination overcome the surface film.
I debunk a common myth: “stain‑less” means more resistant, not impossible to corrode. In practice, exposure and handling set the outcome.
Common misconceptions I still hear
People assume alloy choice alone prevents trouble. That is false. Poor design, trapped salts, and embedded iron cause most problems.
When “stainless” isn’t stain-less: scenarios that trigger attack
Chloride-rich air, de‑icing salts, and pool chemicals quickly challenge the passive oxide. Tight crevices and lap joints starve oxygen and trap corrosives.
- Shop errors: carbon‑brushes or grinding dust embed iron and seed rust.
- Heat tint: high temperatures can scale the film until the surface repassivates.
- Stress cracking: warm chloride service can cause SCC in some austenitic grades.
I inspect for early tea staining, light pits, and discoloration. Routine cleaning and correct material pairing usually keep corrosion in check for most applications.
The science I rely on: composition, microstructure, and properties
Small shifts in composition often decide whether a part survives or fails in chloride service.
I watch key elements closely. Increasing chromium above about 11% builds a more durable passive oxide that boosts corrosion resistance.
Chromium, nickel, molybdenum, and nitrogen: tailoring resistance
Nickel stabilizes the austenitic structure and improves toughness and formability. It also changes how a grade handles stress corrosion.
Molybdenum and nitrogen raise pitting resistance, especially in chloride environments. I pick alloys with extra molybdenum when facing seawater or splash zones.
Magnetism and what it tells me
I use a magnet as a field test. Austenitic parts are usually non‑magnetic; ferritic, martensitic, and duplex show magnetism.
Cold work can make austenitic alloys slightly magnetic at bends or cut edges. That clue helps me match material to service without lab tests.
- Selection tip: read composition, then verify formability and magnetism before finalizing a grade.
- Practical note: microstructure dictates heat‑treat options—martensitic steels can harden, austenitic cannot by simple tempering.
Forms of corrosion I look for on stainless steels
My field checks focus on visible signs that point to specific corrosion modes.

Pitting and crevice corrosion
I watch for small, deep cavities where chloride attack breaks the passive layer. These pits often hide beneath deposits or near welds and fasteners.
Field signs: pinpoint brown spots, localized loss, and deposits in gaps. Mitigation: choose higher PRE alloys such as 316 or duplex, remove deposits, and improve drainage.
Intergranular attack
This occurs when grain boundaries lose chromium after sensitization. The surface may appear sound while toughness drops.
Field signs: edge cracking after heat exposure or welding. Fixes include proper welding practice, post‑weld heat treatment, or selecting low‑carbon or stabilized grades.
Stress corrosion cracking
When tensile stress, chlorides, and heat combine, austenitic stainless can fracture without heavy general corrosion. This phenomenon is particularly concerning in environments where these factors are prevalent, such as coastal areas or industrial settings. To better assess the risk of such failures, engineers often conduct stainless steel weight analysis to evaluate the integrity of structures and components. This analysis helps in understanding how different stressors may affect the longevity and performance of austenitic stainless steels under specific conditions.
Field signs: brittle cracks often starting at welds or machined features. Reduce risk by relieving stress, using duplex or martensitic stainless for the load case, and controlling chloride exposure.
Galvanic corrosion
Dissimilar metals in contact with an electrolyte create accelerated attack on the less noble part. Area ratios matter: small anodes suffer fastest.
Field signs: localized pitting near joints or discolored interfaces. Use isolators, coatings, or match metals and area sizes to avoid rapid loss.
Galling and wear
Adhesive wear destroys the oxide under load, causing seizing of fasteners and moving parts. Austenitic fasteners are common culprits.
Field signs: transfer, scoring, and locked threads. Prevent with different materials in contact, lubrication, or hardened coatings.
- I pair each mode with quick checks and grade/finish choices to reduce risk.
- For more on magnetism and grade clues, see this short guide on magnetism and grade selection.
Key factors that make stainless steel corrode faster
I find that a few predictable conditions repeatedly turn a resistant alloy into a problem part.

Chlorides, acids, and harsh cleaners
Chloride ions promote pitting and stress corrosion cracking. Salt or de‑icing residues concentrate at crevices and start tiny pits.
Strong acids and aggressive cleaners can dissolve the passive oxide and remove the protective layer. That loss reduces corrosion resistance until the surface repassivates.
Oxygen starvation in tight gaps
Crevices and overlapped joints limit oxygen access. Without oxygen, the passive film cannot self‑repair.
Chlorides concentrate in these low‑oxygen spots and drive rapid localized attack, even on high‑PRE alloys.
High temperatures and scaling
Heat tint and scale change surface chemistry. Elevated temperatures thin the protective layer temporarily.
Hot chlorides are more aggressive, so heat plus salts raises the risk of corrosion and cracking.
Surface contamination and embedded iron
Carbon‑tooling debris, grinding dust, and wire‑brush particles embed into the surface and seed rust spots.
Discipline in handling and cleaning stops these seeds before they cause pits.
- Mitigations: upgrade grade for chloride service, improve drainage and joint design, and use electropolished or passivated finishes.
- Work practice: avoid carbon tools, rinse thoroughly, and inspect for heat tint after welding.
- Rule of thumb: multiple factors stack risk—hot chlorides in a crevice is far worse than any single cause.
How I choose the right grade: environments, PRE, and cost
My choice process starts with quantifying pitting risk for the service environment. I use a simple index, compose checks, and then weigh fabrication and budget.

Using the Pitting Resistance Equivalent (PRE)
PRE = %Cr + 3.3×%Mo + 16×%N. Higher PRE predicts better pitting and crevice corrosion resistance. I calculate PRE to compare alloys quickly.
304 vs 316, duplex, and super duplex choices
For coastal or salty exposure, 316 outperforms 304 because molybdenum raises PRE. The extra cost is justified where chloride attack is likely.
When strength and chloride SCC resistance matter, I select duplex stainless steel grades. They combine higher yield strength with better resistance than common austenitic stainless alloys.
For aggressive splash zones or warm brines, super duplex is the step up; it gives the highest localized corrosion resistance in my toolbox.
- I balance PRE with fabrication ease, availability, and budget to find a fit-for-purpose grade.
- Sometimes a smoother finish and better design let a lower-PRE grade perform adequately—except in severe chlorides.
- Decision guide: potable water (low PRE ok), food contact (304/316 choice), marine atmosphere (316 or duplex), full immersion (duplex or super duplex).
Preventing rust on stainless steel: my step-by-step approach
Good corrosion control begins with simple design choices that stop water and salts from lingering. I start at the design stage and push the project toward long service life before worrying about repairs.

Design and material selection to avoid crevices and mixing metals
I eliminate stagnant crevices, specify drainable joints, and avoid dissimilar metals unless isolated. Choosing the right grade for the environment—304, 316, or duplex—matters more than a cheaper panel that traps salts.
Finishes, passivation, and electropolishing for tougher surfaces
Chemical passivation restores a chromium-rich film and helps the oxide self-repair. For harsh service I specify electropolishing to smooth the surface and boost corrosion resistance.
Gaskets, fasteners, and isolators to beat galvanic pairs
I standardize stainless steel components and steel components by compatible family and finish to reduce galvanic risk.
- Use isolators, gaskets, or coatings where metals mix and watch area ratios.
- Prevent galling by pairing dissimilar grades, adding lubrication, or using anti-galling alloys.
- Document cleaners and ban carbon tools near work to avoid embedded iron.
Acceptance criteria: no staining, drainable joints, and threaded fasteners turn freely. I set inspection and cleaning intervals by exposure and include simple field checks to keep corrosion from starting.
Care and maintenance routines I recommend
Small habits keep the protective layer working and stop most problems before they start.
Daily cleaning that preserves the passivation layer
I wipe surfaces each day with a mild detergent and water. I follow with a thorough rinse and dry to support repassivation in air.
After salt or acidic spills on cookware and appliances I clean immediately. Long, damp storage invites tea staining and early corrosion.
Products I use—and what I avoid—on stainless surfaces
- Use: non-chloride, non-abrasive cleaners and microfiber cloths that won’t scratch the finish.
- Avoid: steel wool, carbon pads, and chloride-heavy bleaches that embed iron or attack the oxide.
- Tools: nylon pads or dedicated stainless pads for stubborn marks; rinse well after use.
- Periodic care: deep clean and, if exposed to heavy salts, consider a passivation treatment to restore the layer.

- Spot or streak: clean with mild detergent, rinse, dry.
- Early rust bloom: use a nylon pad, clean, then passivate if staining returns.
- Persistent pits or loss: consult repair and consider replacing the affected materials or moving to a higher‑PRE alloy.
My rule: keep items clean, rinse salts away, dry promptly, and avoid abrasive metals near finished parts to maintain long service and corrosion resistance.
Identifying and treating rust on stainless steel
Quick, accurate diagnosis saves parts and time. I start by deciding if a mark is cosmetic or structural. That choice sets the repair steps and safety checks.
Spotting the type of attack before you act
I look for three common outcomes: light tea staining, contamination from embedded iron, or true pitting and crevice attack.
- Tea staining: brown tint that wipes away with mild detergent and a soft cloth.
- Embedded iron: fine rust lines near welds or cut edges—use an iron remover to decontaminate.
- Pitting/crevice: small, deep holes or trapped deposits that need detailed inspection.
From tea staining to pits: cleaning, pickling, and repassivating
I progress from gentle to stronger treatments. Start with soap, water, and a rinse. If contamination persists, use a dedicated stainless cleaner and an iron decontamination product.
For heat tint, deep pits, or metal loss I recommend pickling gels or baths to remove damaged metal before passivation. After pickling, chemical passivation restores a chromium-rich passivation layer and improves corrosion resistance.
- Electropolishing is worth it when future resistance and finish matter.
- Always rinse, dry, and inspect after treatment.
- Document repairs and schedule follow-ups in harsh environments.
Red flags: deep pits, cracking, or signs of stress corrosion call for metallurgical assessment rather than DIY fixes. For background on whether a surface will fail you can read will stainless steel rust.
Stainless steel in kitchens and cookware: practical realities
Kitchen use concentrates factors—salt, heat, and detergents—that test any cookware’s resistance over time.
I keep routine habits that limit corrosion and protect the cooking surface. Small choices in handling and cleaning prevent most problems and extend service life.
Salt, heat, and dishwashers: how I reduce risks day to day
Adding salt before water boils can leave crystals on a hot pan and start tiny pits. I add salt after the boil when possible to avoid that risk.
Dishwashers are fine for many pans, but I avoid chlorine bleach and rinse away salty residues. I dry items promptly to prevent tea staining and surface spotting.
Cookware grades and finishes that perform under heat
Austenitic stainless alloys like 304 are common in cookware for formability, cleanability, and basic corrosion resistance. For brine cooking or coastal kitchens I upgrade to 316 for added molybdenum and higher pitting resistance.
- Finish matters: polished interiors clean easily; brushed finishes hide wear.
- Preheat and seasoning: warm pans gently, use a light oil layer for nonstick help, and avoid harsh scrubbing.
- Utensils: choose silicone, wood, or quality nylon to avoid gouging the finish and embedding particles.
I remove heat tint and discoloration with mild cleaners or a pickling paste when needed, then passivate the layer gently. These steps mirror practices used in commercial food plants to preserve the oxide and keep cookware safe for food applications.
Industrial and outdoor applications: what changes in the field
When I specify alloys for plants and piers, corrosion control becomes a systems decision, not just a material pick. Exposure, load, and maintenance shape grade choice and detailing.
Marine, chemical processing, and water treatment considerations
I tailor grade selection to splash zones, brine handling, and disinfection chemistry common in water systems. For chloride‑rich service, duplex stainless and super duplex often outlast common austenitic grades because they resist pitting and chloride SCC better.
In chemical plants I match alloys to temp and acid exposure, and I insist on clean finishes and strict welding practice to preserve corrosion resistance.
Structural uses: lean duplex and cost‑effective durability
For structural components I often choose lean duplex. Its higher yield strength lets me use thinner sections and cut weight and cost while keeping adequate corrosion resistance.
That tradeoff lowers lifecycle maintenance in many outdoor builds versus using thicker low‑alloy steels or standard austenitic parts.
Fabrication, fasteners, and maintenance
- I specify weld procedures, post‑weld cleaning, and electropolishing where appearance and long life matter.
- Fastener and gasket choice matters: match compatible metals or add isolators to avoid galvanic traps in wet environs.
- Set inspection intervals and cleaning regimes for coastal sites—regular rinsing of salt deposits prevents tea staining and early pitting.
Cost‑benefit note: upgrading to a duplex grade often raises initial cost but lowers repair and downtime over decades in harsh outdoor or process applications. I weigh life‑cycle cost, not just sticker price, when I recommend materials for long‑lived installations.
When stainless steel meets other metals: stopping galvanic corrosion
When two different metals meet in a wet environment, electrochemistry decides which one pays the price.
I explain the galvanic series so you know why an alloy often becomes the cathode and drives corrosion on the less noble partner. Area ratios and a conductive film of water raise the risk quickly in coastal or humid applications.
Isolation is my first line of defense. I use nonconductive gaskets, sleeves, and polymer spacers to break electrical contact at bolted joints.
Practical measures that work
- Coatings and sealants: apply where isolation isn’t feasible, and check edges regularly for breaches.
- Sacrificial options: zinc‑coated carbon fasteners or dedicated anodes protect the anodic metal in constant exposure.
- Area control: avoid small anodic patches feeding large cathodic surfaces in wet service.
- Standardize combinations: pick compatible fasteners and base materials to reduce mixed‑metal interfaces.
- Inspection schedule: check bolted joints and interfaces in marine or humid sites and record findings.
Document installation practices to prevent hidden crevices and moisture traps. For more on corrosion mechanics and practical fixes, see my deeper guide on stainless steel and rust.
My final take: how to keep stainless steel truly “stainless” today
My final take: here’s the bottom line — protect the passive film, match the grade to the task, and inspect often to keep stainless steel assets performing.
The passive chromium oxide layer heals in air, so good design and simple care preserve corrosion resistance. Choose 304 indoors, 316 near salt, and duplex or precipitation‑hardening grades where strength and chloride exposure combine.
Design out crevices, isolate mixed metals, and favor smooth finishes. Clean daily with non‑chloride products, rinse, and dry to help the oxide self‑repair and cut early corrosion risk.
Quick checklist to use now: match materials to applications, remove contaminants, avoid carbon tools, schedule audits, and replace troubled parts before pits grow.






